The food and drug administration fda or the agency is issuing this final rule revising its medical device and certain biological product labeling regulations to explicitly allow for the optional.
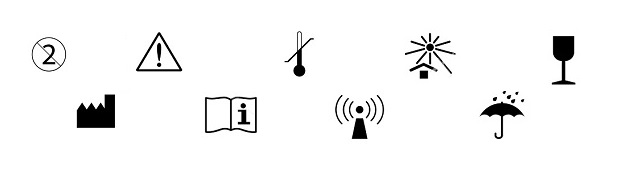
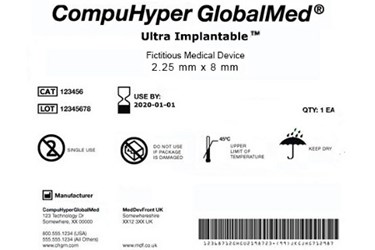
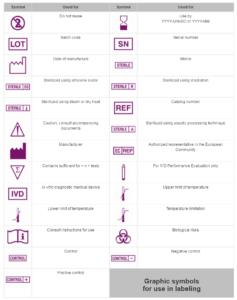
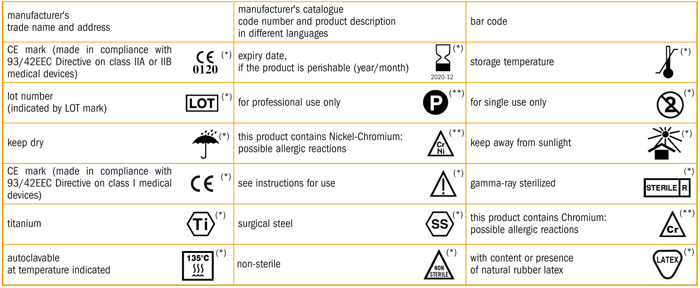
Fda medical device labeling symbols.
801 6 medical devices.
The rule finalizes the fda s 2013 proposed rule and affects biologics ivds and medical devices regulated by 21 cfr parts 660 801 and 809.
Subpart a general labeling provisions 801 1 medical devices.
801 5 medical devices.
The use of symbols in medical device labeling final rule final rule including the requirement for a glossary only applies to symbols that are used to convey information required by or under.
Symbols in medical device labeling can also convey important information.
Introduction to medical device labeling label vs.
801 15 medical devices.
The final rule permits the use of symbols in all medical device labeling without adjacent explanatory text referred to as stand alone symbols if certain requirements are met.
Food and drug administration fda develops and administers regulations under authority granted by laws passed by congress that.
Adequate directions for use.
Name and place of business of manufacturer packer or distributor.
Fda assumes that the labeling of each uniquely labeled medical device can communicate the same information using alone standsymbols or symbols with adjacent explanatory text.
Food and drug administration fda develops and administers regulations under authority granted by laws passed by congress that.
However to be an effective means of communicating information it s critical that symbols on medical devices are.
Prominence of required label statements.
Fda issues final rule on symbols in medical device labeling by suzanne hodsden the fda has released a final rule governing the use of symbols on device labeling that will allow manufacturers to use standalone symbols which have been in use overseas for years without adjacent explanatory text as long as certain conditions are met.
801 3 definitions.
801 4 meaning of intended uses.



.jpg)












